Sublimation Is Defined as a Change of State From
It is not easy to actually see sublimation occurring at least not with ice. Sublimation Noun chemistry a change directly from the solid to the gaseous state without becoming liquid.
Matter Lesson 3 Changes Of State In Matter
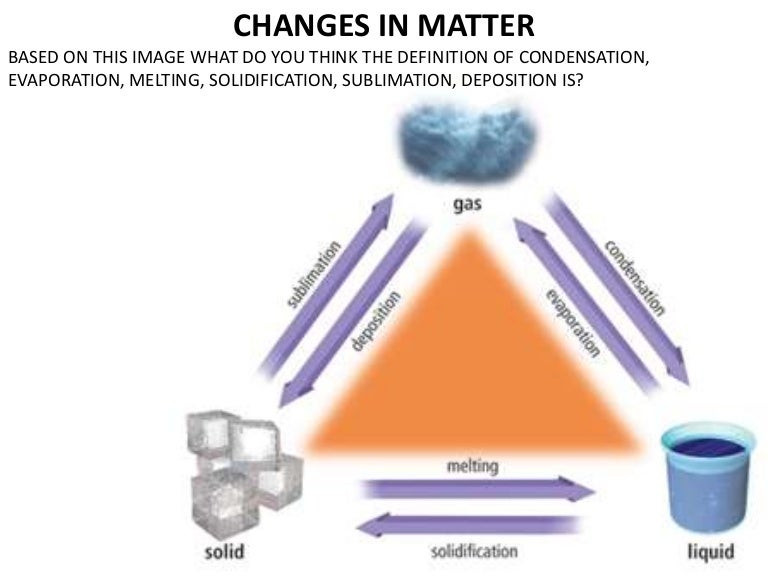
However plasma also is a state of matter so a complete list requires all eight total phase changes.
. It is called an endothermic reaction because of the additional energy required. The opposite process of this where the gas goes directly to the solid phase is called de-sublimation or deposition. Change of state synonyms change of state pronunciation change of state translation English dictionary definition of change of state.
The physical process where matter moves from one state to another. One way to see the results of sublimation is to hang a wet shirt outside on a below-freezing day. With a change in temperature and pressure on a substance it can transform from one state to another as in the following example of water.
The closeness arrangement and motion of the particles in a substance change when it changes state. Matter exists in the familiar states of a solid liquid or gas. Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase.
The process in which solids directly change to gases is defined as sublimation. The phenomenon is the result of vapour pressure and temperature relationships. The most commonly known phase changes are those six between solids liquids and gasses.
Sublimated sublimating sublimates vintr. When the liquid gets converted to gas at all the temperatures it is known as evaporation. Dry ice is a case of solids that undergo sublimation Snow and ice can sublime in the wintertime without melting Mothballs sublime Frozen foods sublime and.
Matter undergoes phase changes or phase transitions from one state of matter to another. Eventually the ice in the shirt will disappear. Sublimation Definition In science sublimation is a distinct type of phase transition during which a matter directly changes from solid to its gaseous form bypassing the liquid state of matter.
It is an endothermic process at pressure and temperature. This endothermic phase transition occurs at temperatures and pressures below the triple point. Below is a complete list of the names of these phase changes.
Then sublimation is defined as heat or energy required to change the state from solid. Freeze-drying of food to preserve it involves sublimation of water. This process specifically occurs at temperature and pressure below the matters or substances triple point.
Sublimation is the method by which the material changes from the frozen solid to the gas without going through the intermediate liquid state. The opposite of sublimation is deposition where water vapor changes directly into icesuch a snowflakes and frost. Sublimation Noun psychology modifying the natural expression of an impulse or instinct especially a sexual one to one that is socially acceptable.
In scientific terms Sublimation is the transition of a substance directly from a solid-state to a gas state. This occurs when solids absorb enough energy to overcome the forces of attraction between them. Sublimation is defined as the process in which the solid-state changes to a gaseous state without changing into a liquid state.
This entry deals with the original meaning of sublimation found in chemistry. Sublimation is changing from a. The term sublimation only applies to physical changes of state and not to the transformation of a.
To modify the natural expression of a primitive. Maharashtra State Board Previous Year Question Paper With Solution for Class 10. Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce.
It is lower than the triple point of substance with its transition phase that refers to the lower pressure at which the substance would remain the. Alteration change modification shift transformation transmutation. How to use sublimate in a sentence.
When some molecules absorb heat energy they are at a much higher energy state than their neighbours hence overcoming the force of attraction and therefore escape into the vapour phase. The main difference between Sublimation and Evaporation is that Sublimation can be defined as a process of changing the solid-state of any matter directly into the gaseous state of that matter there is no existence of liquid phase or state in this process and vice versa while comparatively on the other hand Evaporation can be. Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science.
The meaning of SUBLIMATE is sublime. Chemistry To be transformed directly from the solid to the gaseous state or from the gaseous to the solid state without becoming a liquid. Sublimation vs Evaporation.
It does not pass through the usual liquid state and only occurs are specific temperatures and pressures. An example is the vaporization of frozen carbon dioxide dry ice at ordinary atmospheric pressure and temperature. Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts.
Define change of state. Sublimation in physics conversion of a substance from the solid to the gaseous state without its becoming liquid. Chemistry To cause a solid or gas to sublimate.
Sublimation is defined as the change or transition from the solid phase into the gas phase without entering the liquid phase. Its a general term that is used to describe the solid-to-gas transition and refers to the physical change in state only.

To Observe The Process Of Sublimation Class 5 Chemical Changes States Of Matter Observation
Bioprofe Chemistry Changes Of States Of Matter

Art The Phase Changes Of Matter Include Melting Freezing Evaporation Condensation Deposition And Sublima Matter Science States Of Matter Changes In Matter
No comments for "Sublimation Is Defined as a Change of State From"
Post a Comment